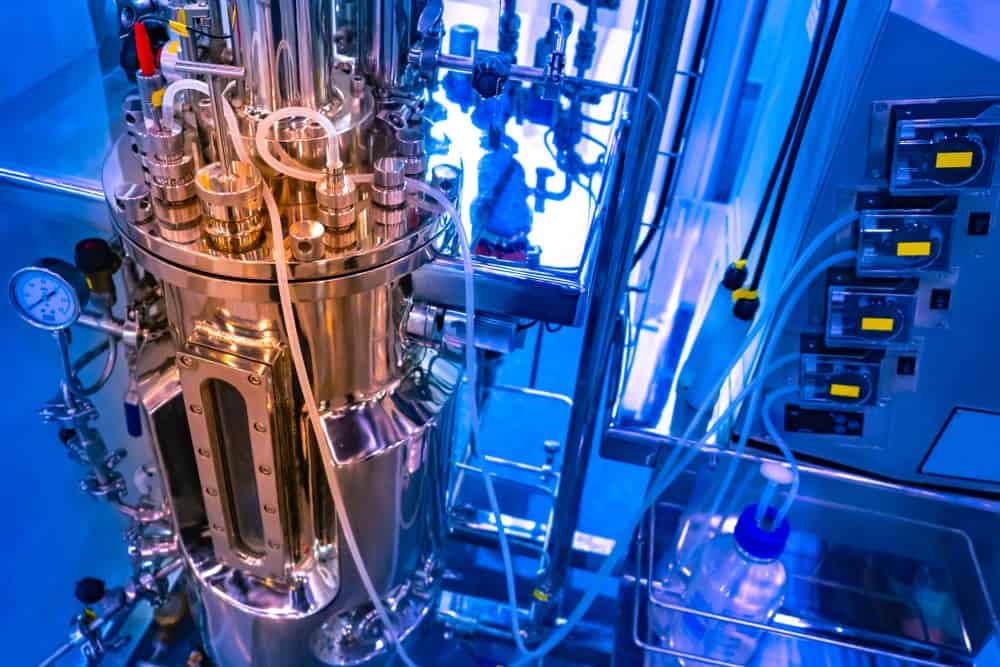
Anything that helps bio-organisms thrive can be considered a bioreactor, from living beings to technological innovations. The bioreactor technology used in pharmaceuticals supports a wide area of biological research, from cell therapy to vaccine development. The engineering of the bioreactor vessel and its integration into the production line is directly related to the success of ideal bioprocess development.
Let us take a detailed look at the bioreactors in the pharmaceutical industry.
Bioreactors for Different Scales of Pharmaceutical Production
The term “bioreactor” in the pharmaceutical industry broadly refers to the vessel or container where microorganisms are stored and allowed to grow under ideal process conditions. The production requirement demands the culture of different microorganisms, including bacteria, cells, and algae. These organisms produce biomolecules or biomaterials, which can be used in medical and pharmaceutical industries as part of vaccines, medicine content, or genetic engineering.
The production process can involve the mass production of standard medicines or customized manufacturing for precision medicine development. Different bioreactor types with varying builds and process control are used for each manufacturing process. The prominent ones used in the pharmaceutical industry include:
- Stirred tank bioreactors: Usually made of stainless steel, these bioreactors consist of an impeller that provides the necessary mechanical agitation to homogenize the contained media. The media usually include cells, antibodies, or enzymes.
- Bubble column bioreactors: This bioreactor consists of a sparger placed at the bottom of the column from which the gas is passed onto the liquid media. The bubbles formed thoroughly mix and aerate the media without the requirement of any agitators.
- Air lift bioreactors: This bioreactor is separated into two channels with the help of an in-built draft tube. While one channel (riser) supports the rise of the bubbles, the other channel (downcomer) assists in downward flow, facilitating intense mixing in both directions. These bioreactors are typically used in cell culture applications.
- Fluidized bed bioreactors: This bioreactor passes the fluid through the distributor to the bed of solid media, usually the immobilized cells. As the velocity reaches the fluidization behavior, the solid starts to suspend, causing the mixing of the media.
- Fixed bed bioreactors: Porous or non-porous catalyst pellets are immobilized by packing them into the beds. The aerated fluid medium circulates, which reacts to the catalyst, and the product is released into the fluid. These bioreactors can be used to cultivate specialized cells.
The selection of these bioreactors will vary depending on the type of bioprocessing requirement and the scale of production desired.
Integrating Bioreactors Into the Pharmaceutical Production Line
A typical industrial-scale bioreactor is a large pressure vessel or tank structure attached to the feed lines, sampling ports, control system, and sterilization system. The control system regulates the flow rate of the fluid for mixing and fermentation as well as helps maintain temperature control for proper cultivation. In addition, there are sensors for oxygen monitoring and harvest tubes.
Pharmaceutical production is very demanding, chemically and mechanically, for the processing equipment in use. The material used in the production line, including bioreactor and supporting equipment, should, thus, cover the following criteria –
- The metal should be resistant to corrosion and have a smooth finish to prevent contamination.
- The metal should not be chemically reactive and hinder the pH balance of the bio-mixture.
- When exposed to steam during sterilization, the material should be able to resist its high pressure and temperature.
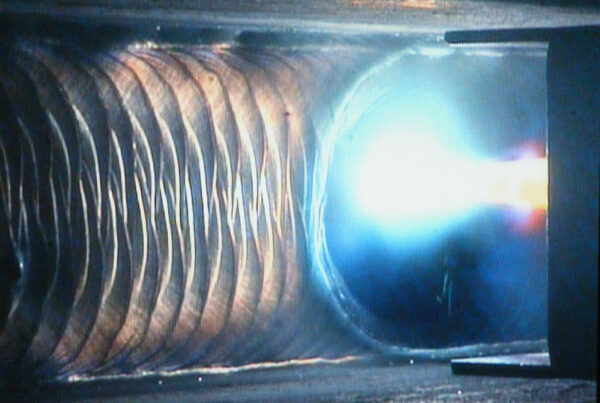
Despite the material selection, the fabrication process is where the challenge lies for bioreactors in the pharmaceutical industry. The precision heat and mass transfer that the process requires may be lost or compromised due to defective fabrication. Defective welds can lead to leaks which will hinder the precision of fluid input and lead to contamination which will not just fail the production process but can also be hazardous to human health.
Fabrication standards such as BPVC (Boiler and Pressure Vessel Code) and BPE (Bioprocessing Equipment) as set by ASME (American Society of Mechanical Engineers) are important baselines to follow when fabricating bioreactors for the pharmaceutical industry. In addition, with sanitary standards set by the American Welding Society (AWS); D18, manufacturers can cover the hygiene requirement while welding.
A Simple Solution to a Complex Problem
Most manufacturers prefer the use of orbital GTAW to fabricate sanitary bioreactors. The flexibility of orbital GTAW will facilitate welding of any configuration type from any position. In addition, the weld parameter control can help the formation of smooth and defect-free welds. The process is also ideal for welding stainless steel, the metal of choice for pharmaceutical bioreactors.
The consistent and clean weld achieved with orbital GTAW is a simple and efficient solution to integrating high-quality bioreactors in the pharmaceutical industry.
For large or small welding projects, orbital welding techniques promote
Arc Machines, Inc. is a leading provider of orbital GTAW solutions that will help you meet welding standards required for fabricating bioreactors in the pharmaceutical industry. For inquiries regarding products, contact sales@arcmachines.com. For service inquiries, contact service@arcmachines.com. Contact us to arrange a meeting. Arc Machines welcomes the opportunity to discuss your specific needs. .