Bioreactors are specialized pressure vessels used in pharmaceutical, food, and medical industries to provide a biologically optimal environment for cell or bacterial growth. For example, this cellular or bacterial growth has been an essential part of vaccine or insulin production in biopharmaceuticals. Moreover, in recent years, this mechanism has also been introduced for growing synthetic meat in a facility.
The sanitation requirement is one common factor in the bioreactor uses and production mechanisms across these different industries and applications. Contamination can occur easily and often if the fabrication of the bioreactor is not performed to sanitary standards. However, bioreactor manufacturing processes can achieve sanitary requirements with the help of safe and reliable orbital welding equipment.
Sanitary Requirement in Bioreactor Manufacturing
The sanitary standards required for food and pharmaceuticals are continuously evolving to prevent the potential contamination of products meant for human consumption. In the manufacturing sector, this is accomplished by setting material selection and welding standards when fabricating tubes, pipes, and vessels.
The pressure vessel, or “bioreactor,” is where the microorganisms are stored and processed. This is also where chemical reactions occur under ideal process conditions to produce the biomolecules used for cellular engineering. Unfortunately, the presence of foreign bacteria during cultivation in the bioreactor can lead to reduced yield and, in many cases, the formation of harmful products that may be potentially harmful to human lives. One way to avoid this situation is through sanitary manufacturing.
The fabrication standards set by ASME (American Society of Mechanical Engineers) focus not just on sanitary pressure vessel manufacturing but also on systems such as CIP (cleaning-in-place) and SIP (sterilize-in-place) that concentrate on meeting standardized cleaning efficiency within a bioreactor. Here are a few sanitary standards that are relevant for bioreactor manufacturing–
- ASME BPVC (Boiler and Pressure Vessel Code) Section VIII: Design, construction, operation, and inspection of pressure vessels.
- ASME BPE (Bioprocessing Equipment): Design, installation, and inspection of equipment such as tanks and pressure vessels used in the bioprocessing industries.
- American Welding Society (AWS) D18.3 Specification for Welding of Tanks, Vessels, and Other Equipment in Sanitary (Hygienic) Applications
Let’s discuss the relevant welding standards and the role of orbital welding in meeting the required sanitary standards in bioreactor manufacturing.
Orbital Welding Helps To Meet Safety Standards
Inaccurate welding provides many chances for contamination.
- Foreign inclusions can be introduced when welding without proper shielding provisions for the weld puddle.
- Improper welding creates cracks and burrs, which can be harder to detect and repair or clean compared to other smooth surfaces. These defects can produce a breeding ground for contaminants and also make the bioreactor unsafe for operation.
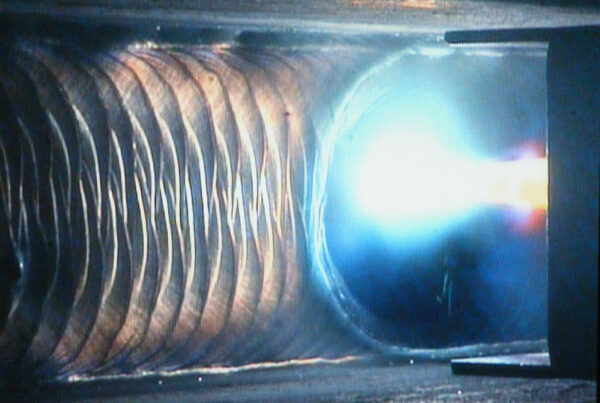
For safe and clean welding of bioreactors, connecting tubes, and piping systems, orbital welding is the right choice. Orbital GTAW, in particular, provides solutions that address the issues mentioned above with —
- Controlled heat input
- Shielding gas protecting the weld puddle
- On-the-fly optimization of weld parameter
- Remote weld monitoring
- Flexible and precise welding
In most cases, the metallurgical choice when manufacturing these bioreactors is stainless steel. Stainless steel is corrosion-resistant, possesses excellent mechanical strength, and provides smooth finishing. However, welding metal with high-chromium content is hazardous for operators due to exposure to hexavalent chromium. Orbital GTAW eliminates this issue by keeping the operators in the monitoring role, which can be performed remotely with advanced technology.
With good welding practice and advanced welding equipment, it is possible to manufacture a leak-free, clean bioreactor that can carry out sanitary production.
Providing Safe Environment for Bioreactor Manufacturing
As a clean and precise process, orbital welding is ideal for fabricating sanitary bioreactors. With shielding and process control, orbital welding can prevent potential contamination by providing a safe environment for creating smooth, defect-free welds. However, be sure to note the degree of preparation and cleanliness required of weld heads and machines when exploring sanitary welding.
Surface cleaning and workpiece preparation as well as standard procedures for cleaning the welding machine and weld head are essential to eliminate possibilities for defects and contamination. By maintaining standards for metallurgy, weld preparation, sanitation, and sanitary welding equipment for orbital welding, manufacturers can ensure a clean environment for bioreactor manufacturing.
Arc Machines, Inc., a leader in orbital welding solutions, will provide you with easy-to-clean and maintain weld heads and machines so you can meet the sanitary requirement during bioreactor manufacturing. For inquiries regarding products, contact sales@arcmachines.com. For service inquiries, contact service@arcmachines.com. Contact us to arrange a meeting. Arc Machines welcomes the opportunity to discuss your specific needs.